14. Solutions
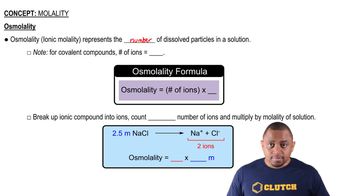
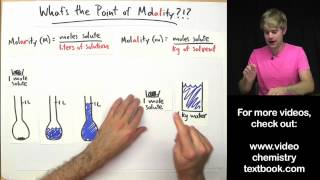
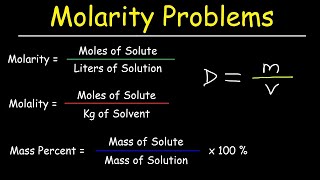
Molality

Get help from an AI Tutor
Ask a question to get started.
Problem 98
Textbook Question
Textbook QuestionAcetonitrile 1CH3CN2 is a polar organic solvent that dissolves a wide range of solutes, including many salts. The density of a 1.80 M LiBr solution in acetonitrile is 0.826 g>cm3. Calculate the concentration of the solution in (a) molality,
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
419
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos