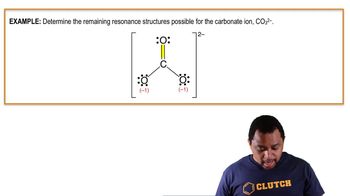
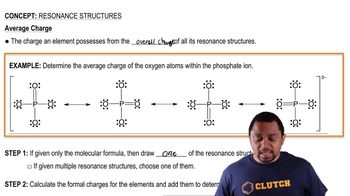
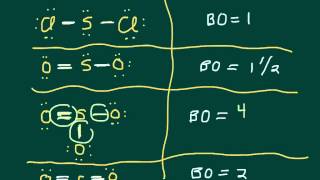
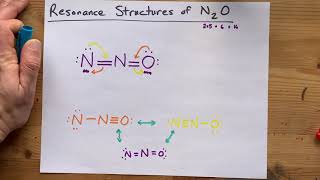
11. Bonding & Molecular Structure
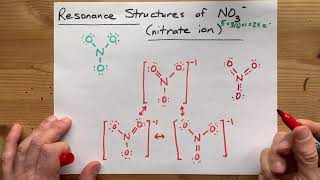
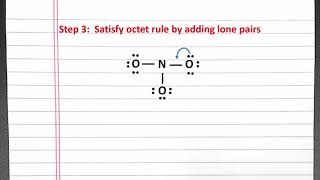
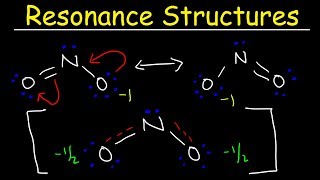
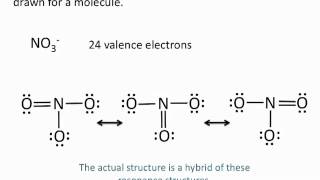
Resonance Structures

Get help from an AI Tutor
Ask a question to get started.
Problem 92b
Textbook Question
Textbook QuestionDiazomethane is a highly poisonous, explosive compound because it readily evolves N2. Diazomethane has the following composition by mass: 28.57% C; 4.80% H; and 66.64% N. The molar mass of diazomethane is 42.04 g/mol. Find the molecular formula of diazomethane, draw its Lewis structure, and assign formal charges to each atom. Why is diazomethane not very stable? Explain.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
8mPlay a video:
546
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos