13. Liquids, Solids & Intermolecular Forces
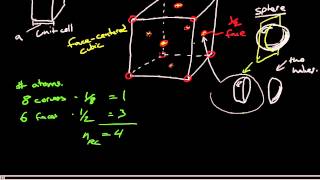
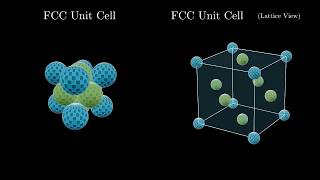
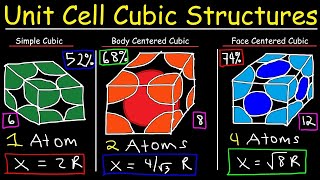
Face Centered Cubic Unit Cell

Get help from an AI Tutor
Ask a question to get started.
Problem 141
Textbook Question
Textbook QuestionThe mineral wustite is a nonstoichiometric iron oxide with the empirical formula FexO, where x is a number slightly less than 1. Wustite can be regarded as an FeO in which some of the Fe sites are vacant. It has a density of 5.75 g>cm3, a cubic unit cell with an edge length of 431 pm, and a facecentered cubic arrangement of oxygen atoms. (c) Each Fe atom in wustite is in either the +2 or the +3 oxidation state. What percent of the Fe atoms are in the +3 oxidation state?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
12mPlay a video:
515
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 7 videos