14. Solutions
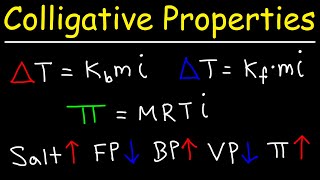
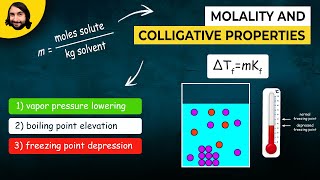
Freezing Point Depression

Get help from an AI Tutor
Ask a question to get started.
Problem 89
Textbook Question
Textbook QuestionWhat mass of salt (NaCl) should you add to 1.00 L of water in an ice cream maker to make a solution that freezes at -10.0 °C? Assume complete dissociation of the NaCl and density of 1.00 g>mL for water.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
2388
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 7 videos