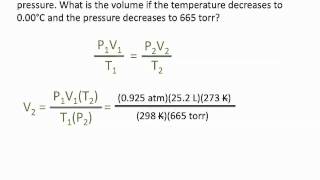
7. Gases
Standard Temperature and Pressure

Get help from an AI Tutor
Ask a question to get started.
Problem 5
Textbook Question
Textbook QuestionPropane gas 1C3H82 is often used as fuel in rural areas. How many liters of CO2 are formed at STP by the complete combustion of the propane in a container with a volume of 15.0 L and a pressure of 4.50 atm at 25.0 °C? The equation for the combustion of propane is: C3H81g2 + 5 O21g2¡3 CO21g2 + 4 H2O1l2 (LO 10.4, 10.5) (a) 61.8 L (b) 186 L (c) 20.6 L (d) 2.21 * 103 L
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
374
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos