7. Gases
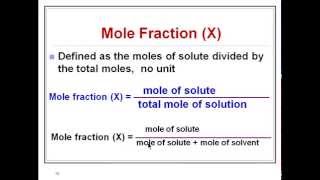
Mole Fraction

Get help from an AI Tutor
Ask a question to get started.
Problem 137
Textbook Question
Textbook QuestionThe apparatus shown consists of three temperature-jacketed 1.000-L bulbs connected by stopcocks. Bulb A contains a mixture of H2O1g2, CO21g2, and N21g2 at 25 °C and a total pressure of 564 mm Hg. Bulb B is empty and is held at a temperature of -70 °C. Bulb C is also empty and is held at a temperature of 190 °C. The stopcocks are closed, and the volume of the lines connecting the bulbs is zero. CO2 sublimes at -78 °C, and N2 boils at -196 °C.

 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
214
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 7 videos