19. Chemical Thermodynamics
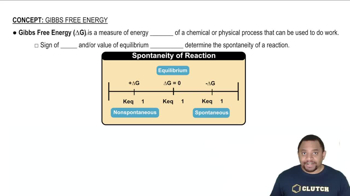
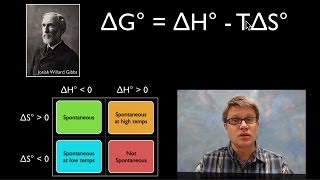
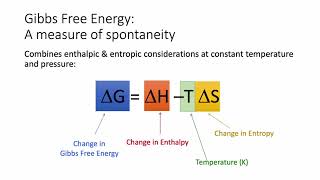
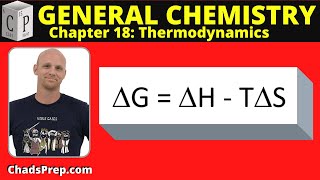
Gibbs Free Energy

Get help from an AI Tutor
Ask a question to get started.
Problem 91a
Textbook Question
Textbook QuestionLiving organisms use energy from the metabolism of food to create an energy-rich molecule called adenosine triphosphate (ATP). The ATP acts as an energy source for a variety of reactions that the living organism must carry out to survive. ATP provides energy through its hydrolysis, which can be symbolized as follows: ATP(aq) + H2O(l ) ¡ ADP(aq) + Pi(aq) ΔGrxn ° = -30.5 kJ where ADP represents adenosine diphosphate and Pi represents an inorganic phosphate group (such as HPO42 - ). b. The free energy obtained from the oxidation (reaction with oxygen) of glucose (C6H12O6) to form carbon dioxide and water can be used to re-form ATP by driving the given reaction in reverse. Calculate the standard free energy change for the oxidation of glucose and estimate the maximum number of moles of ATP that can be formed by the oxidation of one mole of glucose.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
981
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos