17. Acid and Base Equilibrium
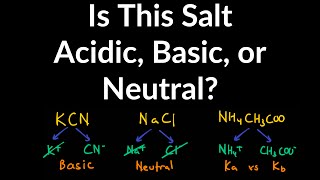
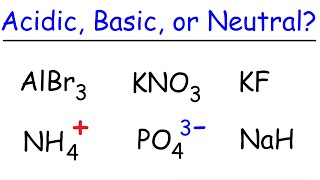
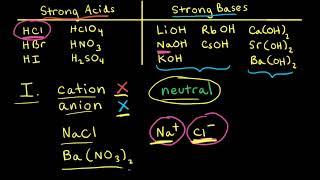
Ionic Salts

Get help from an AI Tutor
Ask a question to get started.
Multiple Choice
Multiple ChoiceDetermine the pH of a 0.55 M NaCN solution. The Ka of hydrocyanic acid, HCN, is 4.9 x 10-10.
A
3.47
B
11.53
C
4.78
D
7.00
1440
views
1
rank
11
comments
Related Videos
Related Practice
Showing 1 of 14 videos