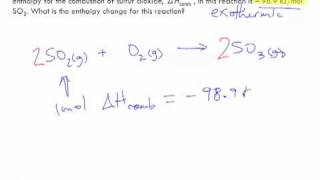8. Thermochemistry
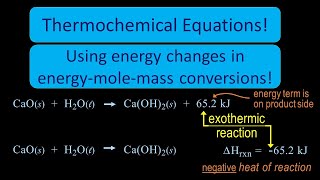
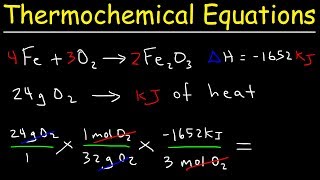
Thermochemical Equations

Get help from an AI Tutor
Ask a question to get started.
Problem 5
Textbook Question
Textbook QuestionIsooctane is the primary component of gasoline and burns in air to produce water rand carbon dioxide 2 C8H18(g) + 25 O2(g) → 16 CO2(g) + 18 H2O(l) ΔH°= —10,940 kJ How much energy is released if 100.0 mL of isooctane (density = 0.690 g/mL) are burned (a) 3300 kJ (b) 6620 kJ (c) 6950 kJ (d) 3.02 kJ
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
1231
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos