14. Solutions
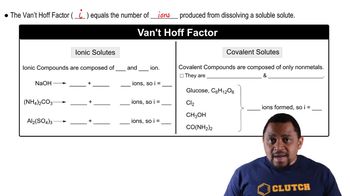
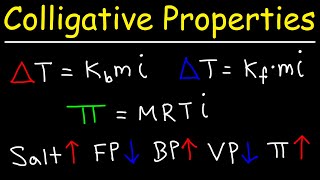
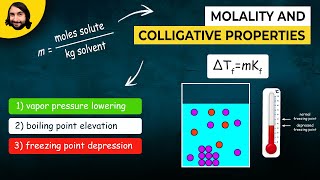
The Colligative Properties

Get help from an AI Tutor
Ask a question to get started.
Problem 73
Textbook Question
Textbook QuestionA solution contains 50.0 g of heptane (C7H16) and 50.0 g of octane (C8H18) at 25 °C. The vapor pressures of pure heptane and pure octane at 25 °C are 45.8 torr and 10.9 torr, respectively. Assuming ideal behavior, answer the following: d. Why is the composition of the vapor different from the composition of the solution?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
962
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos