7. Gases
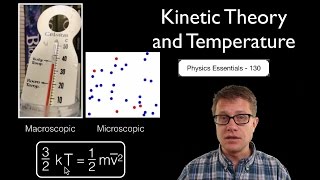
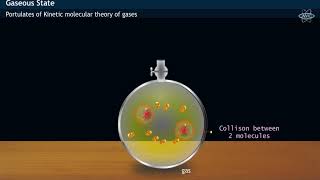
Kinetic Molecular Theory

Get help from an AI Tutor
Ask a question to get started.
Problem 13a
Textbook Question
Textbook QuestionIdentify the true statement about deviations from ideal gas behavior. (LO 10.12) (a) The attractive forces between gas particles cause the true volume of the sample to be larger than predicted by the ideal gas law. (b) The attractive forces between gas particles most influence the volume of a sample at low pressure. (c) The volume of the gas particles themselves most influences the volume of the sample at low pressure. (d) The volume of the gas particles themselves causes the true volume of the sample to be larger than predicted by the ideal gas law.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
508
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 14 videos