15. Chemical Kinetics
Reaction Mechanism

Get help from an AI Tutor
Ask a question to get started.
Problem 105b
Textbook Question
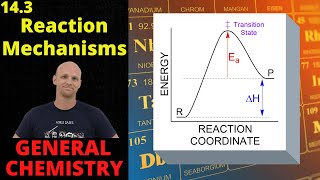
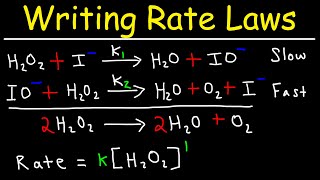
Textbook QuestionConsider the gas-phase reaction: H2( g) + I2( g)¡2 HI( g) The reaction was experimentally determined to be first order in H2 and first order in I2. Consider the proposed mechanisms. Proposed mechanism I: H2( g) + I2( g)¡2 HI( g) Single step Proposed mechanism II: I2(g) Δk1k-12 I(g) Fast H2( g) + 2 I( g) ¡k22 HI( g) Slow b. What kind of experimental evidence might lead you to favor mechanism II over mechanism I?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
1215
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos