10. Periodic Properties of the Elements
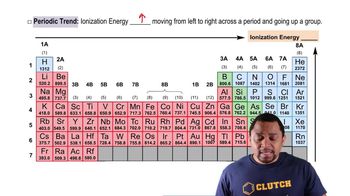
Periodic Trend: Ionization Energy

Get help from an AI Tutor
Ask a question to get started.
Problem 111a
Textbook Question
Textbook QuestionOne way to measure ionization energies is ultraviolet photoelectron spectroscopy (PES), a technique based on the photoelectric effect. (Section 6.2) In PES, monochromatic light is directed onto a sample, causing electrons to be emitted. The kinetic energy of the emitted electrons is measured. The difference between the energy of the photons and the kinetic energy of the electrons corresponds to the energy needed to remove the electrons (that is, the ionization energy). Suppose that a PES experiment is performed in which mercury vapor is irradiated with ultraviolet light of wavelength 58.4 nm. (d) Using Figure 7.10, determine which of the halogen elements has a first ionization energy closest to that of mercury.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
6mPlay a video:
710
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 14 videos