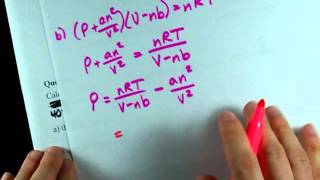7. Gases
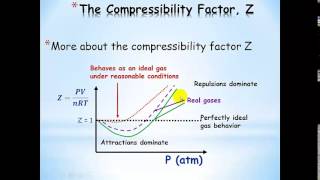
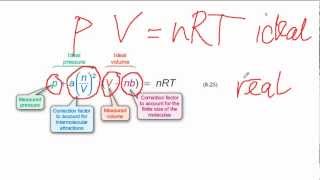
Van der Waals Equation

Get help from an AI Tutor
Ask a question to get started.
Problem 116
Textbook Question
Textbook QuestionIt turns out that the van der Waals constant b equals four times the total volume actually occupied by the molecules of a mole of gas. Using this figure, calculate the fraction of the volume in a container actually occupied by Ar atoms (b) at 20.27 MPa pressure and 0 °C. (Assume for simplicity that the ideal-gas equation still holds.)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
8mPlay a video:
1160
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos