10. Periodic Properties of the Elements
Periodic Trend: Cumulative

Get help from an AI Tutor
Ask a question to get started.
Problem 100
Textbook Question
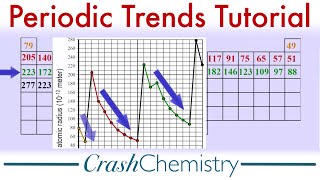
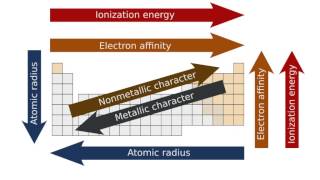
Textbook QuestionMany early chemists noted a diagonal relationship among ele-ments in the periodic table, whereby a given element is some-times more similar to the element below and to the right than it is to the element directly below. Lithium is more similar to magnesium than to sodium, for example, and boron is more similar to silicon than to aluminum. Use your knowledge about the periodic trends of such properties as atomic radii and Zeff to explain the existence of diagonal relationships.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
6mPlay a video:
273
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos