16. Chemical Equilibrium
ICE Charts

Get help from an AI Tutor
Ask a question to get started.
Problem 96
Textbook Question
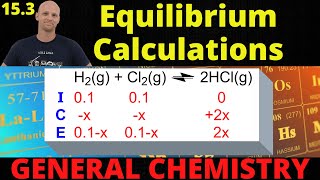
Textbook QuestionThe following equilibria were measured at 823 K: CoO1s2 + H21g2 ΔCo1s2 + H2O1g2 Kc = 67 H21g2 + CO21g2 ΔCO1g2 + H2O1g2 Kc = 0.14 (d) If the reaction vessel from part (c) is heated to 823 K and allowed to come to equilibrium, how much CoO1s2 remains?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
6mPlay a video:
425
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos