12. Molecular Shapes & Valence Bond Theory
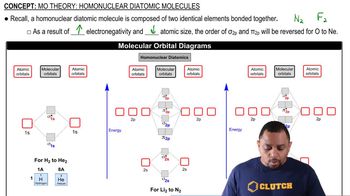
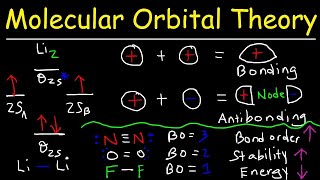
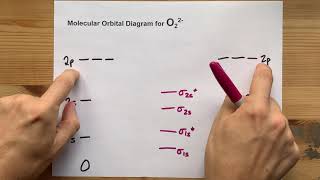
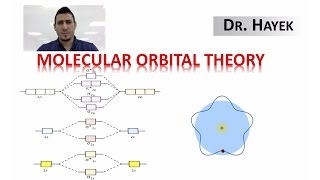
MO Theory: Homonuclear Diatomic Molecules

Get help from an AI Tutor
Ask a question to get started.
Problem 106
Textbook Question
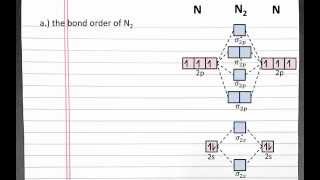
Textbook QuestionAt high temperatures, sulfur vapor is predominantly in the form of S21g2 molecules. (a) Assuming that the molecular orbitals for third-row diatomic molecules are analogous to those for second-row molecules, construct an MO diagram for the valence orbitals of S21g2.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
321
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos