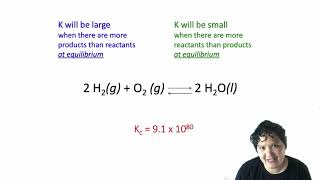16. Chemical Equilibrium
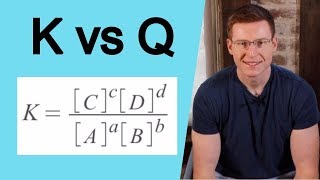
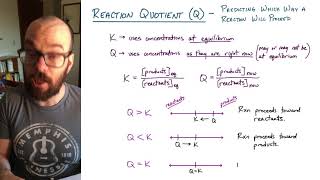
Reaction Quotient

Get help from an AI Tutor
Ask a question to get started.
Problem 83
Textbook Question
Textbook QuestionNitric oxide (NO) reacts readily with chlorine gas as follows: 2 NO1g2 + Cl21g2 Δ 2 NOCl1g2 At 700 K, the equilibrium constant Kp for this reaction is 0.26. Predict the behavior of each of the following mixtures at this temperature and indicate whether or not the mixtures are at equilibrium. If not, state whether the mixture will need to produce more products or reactants to reach equilibrium. (b) PNO = 0.12 atm, PCl2 = 0.10 atm, PNOCl = 0.050 atm
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
602
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos