3. Chemical Reactions
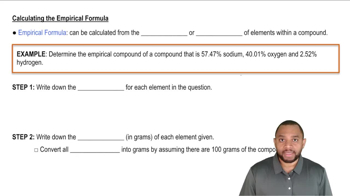
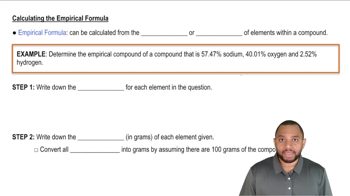
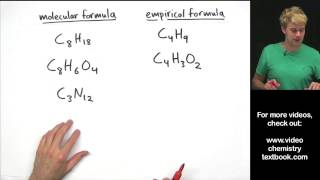
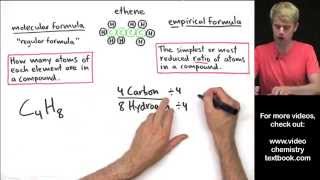
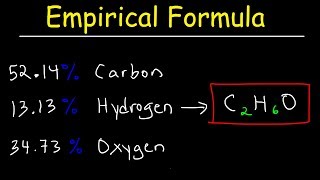
Empirical Formula

Get help from an AI Tutor
Ask a question to get started.
Problem 120
Textbook Question
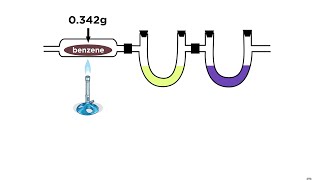
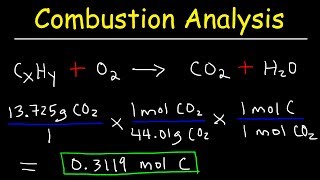
Textbook QuestionAn herbicide is found to contain only C, H, N, and Cl. The complete combustion of a 100.0-mg sample of the herbicide in excess oxygen produces 83.16 mL of CO2 and 73.30 mL of H2O vapor expressed at STP. A separate analysis shows that the sample also contains 16.44 mg of Cl. (c) What other information would you need to know about this compound to calculate its true molecular formula?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
512
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos