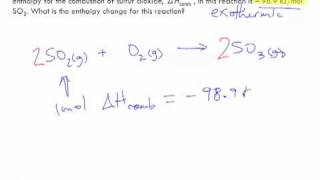8. Thermochemistry
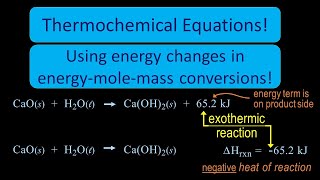
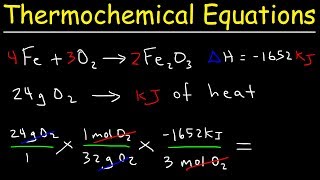
Thermochemical Equations

Get help from an AI Tutor
Ask a question to get started.
Problem 72c
Textbook Question
Textbook QuestionThe combustion of one mole of liquid octane, CH3(CH2)6CH3, produces 5470 kJ of heat. Calculate how much heat is produced if 1.000 gallon of octane is combusted. See Exercise 3.68 for necessary information about octane.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
2143
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos