11. Bonding & Molecular Structure
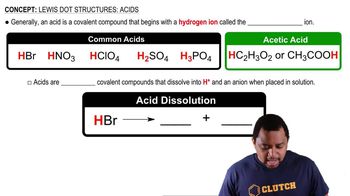
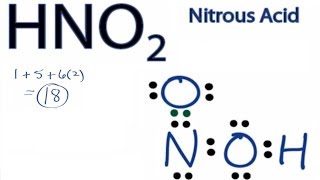
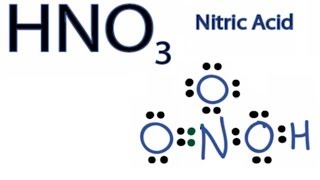
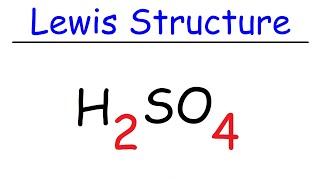
Lewis Dot Structures: Acids

Get help from an AI Tutor
Ask a question to get started.
Problem 107b
Textbook Question
Textbook QuestionWhite phosphorus reacts spontaneously with the oxygen in air to form P4O6. (b) When P4O6 is dissolved in water, it produces a H3PO3 molecule. H3PO3 has two forms, P forms 3 covalent bonds in the first form and P forms 5 covalent bonds in the second form. Draw two possible Lewis structures of H3PO3.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
549
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos