11. Bonding & Molecular Structure
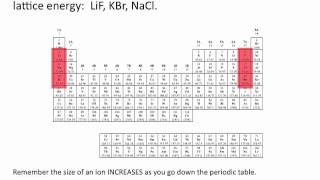
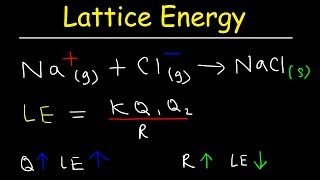
Lattice Energy

Get help from an AI Tutor
Ask a question to get started.
Problem 45
Textbook Question
Textbook QuestionThe lattice energy of CsF is -744 kJ>mol, whereas that of BaO is -3029 kJ>mol. Explain this large difference in lattice energy.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
1793
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos