19. Chemical Thermodynamics
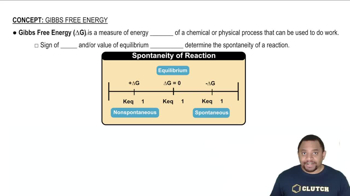
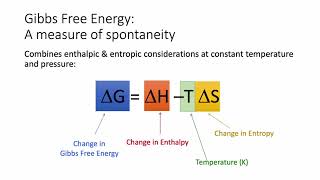
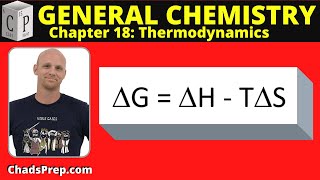
Gibbs Free Energy

Get help from an AI Tutor
Ask a question to get started.
Problem 102a
Textbook Question
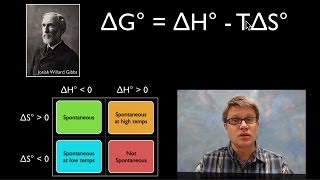
Textbook QuestionEthanol is manufactured in industry by the hydration of ethylene: Using the data in Appendix B, calculate ∆G° and show that this reaction is spontaneous at 25 °C. Why does this reaction become nonspontaneous at higher temperatures? Estimate the temperature at which the reaction becomes nonspontaneous.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
7mPlay a video:
367
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos