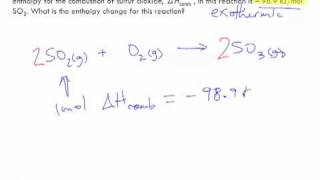8. Thermochemistry
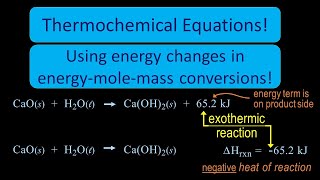
Thermochemical Equations

Get help from an AI Tutor
Ask a question to get started.
Problem 64
Textbook Question
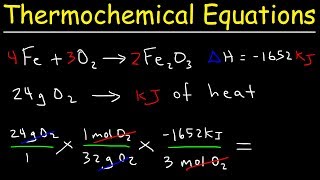
Textbook QuestionCharcoal is primarily carbon. Determine the mass of CO2 produced by burning enough carbon (in the form of charcoal) to produce 5.00 * 102 kJ of heat. C(s) + O2( g)¡CO2( g) ΔH °rxn = -393.5 kJ
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
2995
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos