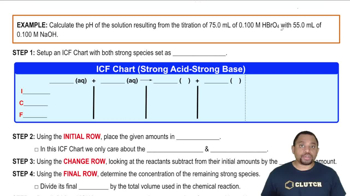
18. Aqueous Equilibrium
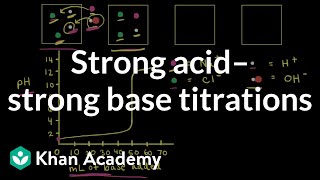
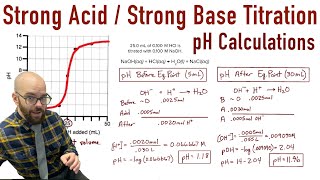
Titrations: Strong Acid-Strong Base

Get help from an AI Tutor
Ask a question to get started.
Problem 63a
Textbook Question
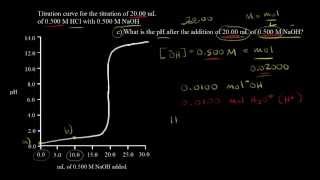
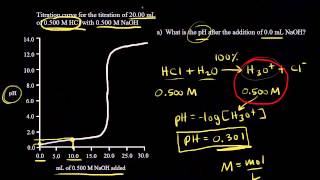
Textbook QuestionTwo 20.0-mL samples, one 0.200 M KOH and the other 0.200 M CH3NH2, are titrated with 0.100 M HI. b. Is the pH at the equivalence point for each titration acidic, basic, or neutral?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
299
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos