8. Thermochemistry
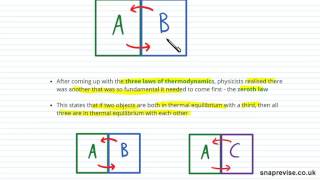
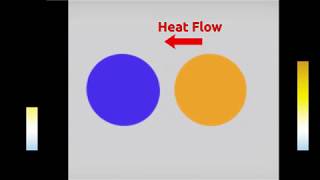
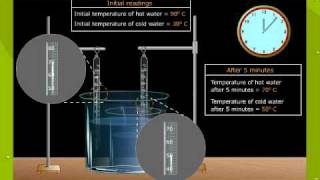
Thermal Equilibrium

Get help from an AI Tutor
Ask a question to get started.
Problem 102
Textbook Question
Textbook QuestionDry ice is solid carbon dioxide. Instead of melting, solid carbon dioxide sublimes according to the equation: CO2(s)¡CO2( g) ◀ When carbon dioxide sublimes, the gaseous cO2 is cold enough to cause water vapor in the air to condense, forming fog. When dry ice is added to warm water, heat from the water causes the dry ice to sublime more quickly. The evaporating carbon dioxide produces a dense fog often used to create special effects. In a simple dry ice fog machine, dry ice is added to warm water in a Styrofoam cooler. The dry ice produces fog until it evaporates away, or until the water gets too cold to sublime the dry ice quickly enough. Suppose that a small Styrofoam cooler holds 15.0 L of water heated to 85 °C. Use standard enthalpies of formation to calculate the change in enthalpy for dry ice sublimation, and calculate the mass of dry ice that should be added to the water so that the dry ice completely sublimes away when the water reaches 25 °C. Assume no heat loss to the surroundings. (The ΔH °f for CO2(s) is -427.4 kJ/mol.)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
1122
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos