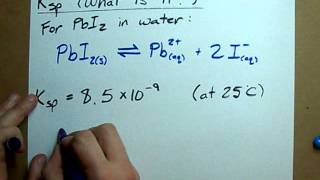18. Aqueous Equilibrium
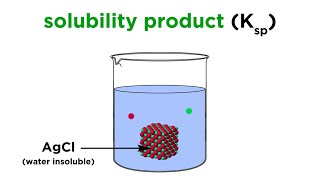
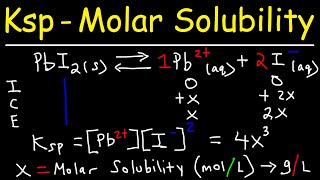
Solubility Product Constant: Ksp

Get help from an AI Tutor
Ask a question to get started.
Problem 92
Textbook Question
Textbook QuestionConsider the compounds with the generic formulas listed and their corresponding molar solubilities in pure water. Which compound has the smallest value of Ksp? a. AX; molar solubility = 1.35 * 10 - 4 M b. AX2; molar solubility = 2.25 * 10- 4 M c. A2X; molar solubility = 1.75 * 10 - 4 M
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
715
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos