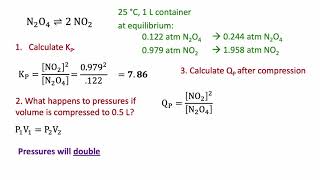
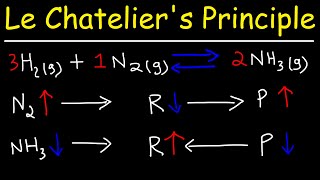
16. Chemical Equilibrium
Le Chatelier's Principle

Get help from an AI Tutor
Ask a question to get started.
Problem 62b
Textbook Question
Textbook QuestionConsider the reaction 4 NH31g2 + 5 O21g2 Δ 4 NO1g2 + 6 H2O1g2, H = -904.4 kJ Does each of the following increase, decrease, or leave unchanged the yield of NO at equilibrium? (f) increase temperature.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
281
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos