16. Chemical Equilibrium
ICE Charts

Get help from an AI Tutor
Ask a question to get started.
Problem 145
Textbook Question
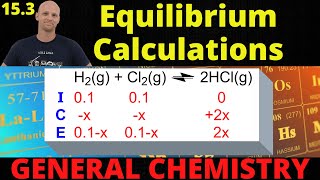
Textbook QuestionHeavy water, symbolized D2O 1D = 2H2 finds use as a neutron moderator in nuclear reactors. In a mixture with ordinary water, exchange of isotopes occurs according to the following equation: H2O + D2O ∆ 2 HDO Kc = 3.86 at 298 K When 1.00 mol of H2O is combined with 1.00 mol of D2O, what are the equilibrium amounts of H2O, D2O, and HDO (in moles) at 298 K? Assume the density of the mixture is constant at 1.05 g>cm3.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
150
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos