16. Chemical Equilibrium
ICE Charts

Get help from an AI Tutor
Ask a question to get started.
Problem 86a
Textbook Question
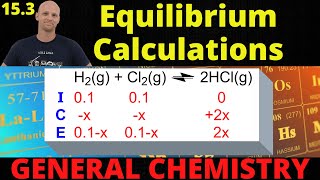
Textbook QuestionThe equilibrium constant Kc for C1s2 + CO21g2Δ 2 CO1g2 is 1.9 at 1000 K and 0.133 at 298 K. (b) If excess C is allowed to react with 25.0 g of CO2 in a 3.00-L vessel at 1000 K, how many grams of C are consumed?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
759
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos