2. Atoms & Elements
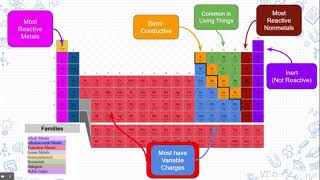
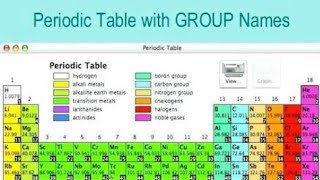
Periodic Table: Group Names

Get help from an AI Tutor
Ask a question to get started.
Problem 101
Textbook Question
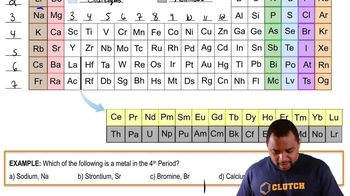
Textbook QuestionThe first atoms of seaborgium (Sg) were identified in 1974. The longest-lived isotope of Sg has a mass number of 266. (b) Atoms of Sg are very unstable, and it is therefore difficult to study this element's properties. Based on the position of Sg in the periodic table, what element should it most closely resemble in its chemical properties?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
371
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos