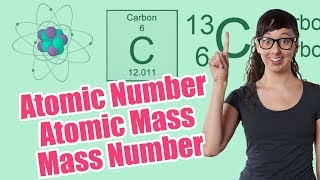2. Atoms & Elements
Atomic Mass

Get help from an AI Tutor
Ask a question to get started.
Problem 121a
Textbook Question
Textbook QuestionA sample of naturally occurring silicon consists of 28^Si (27.9769), 29^Si (28.9765), and 30^Si (29.9738). If the atomic weight of silicon is 28.0855 and the natural abundance of 29^Si is 4.68%, what are the natural abundances of 28^Si and 30^Si?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
9mPlay a video:
545
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 16 videos