10. Periodic Properties of the Elements
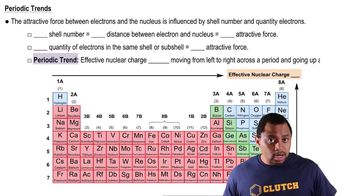
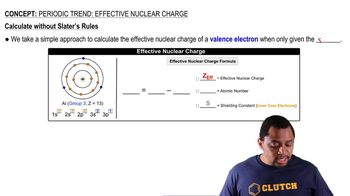
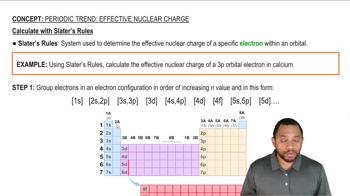
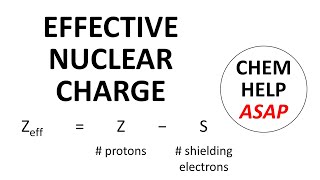
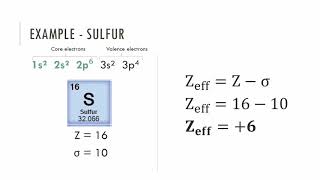
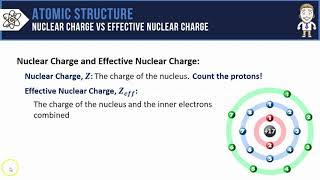
Periodic Trend: Effective Nuclear Charge

Get help from an AI Tutor
Ask a question to get started.
Problem 59c
Textbook Question
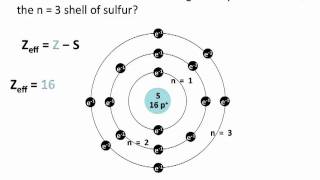
Textbook QuestionIf core electrons completely shielded valence electrons from nuclear charge (i.e., if each core electron reduced nuclear charge by 1 unit) and if valence electrons did not shield one another from nuclear charge at all, what would be the effective nuclear charge experienced by the valence electrons of each atom? a. K
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
461
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos