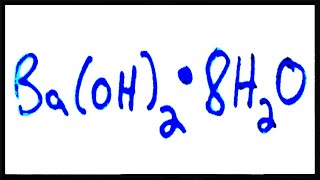3. Chemical Reactions
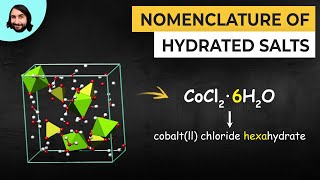
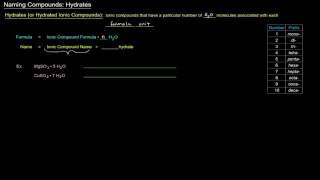
Naming Ionic Hydrates

Get help from an AI Tutor
Ask a question to get started.
Problem 59
Textbook Question
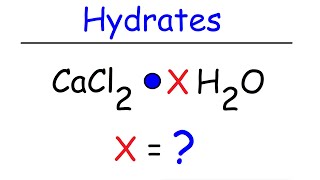
Textbook QuestionWashing soda, a compound used to prepare hard water for washing laundry, is a hydrate, which means that a certain number of water molecules are included in the solid structure. Its formula can be written as Na2CO3 # xH2O, where x is the number of moles of H2O per mole of Na2CO3. When a 2.558-g sample of washing soda is heated at 125 C, all the water of hydration is lost, leaving 0.948 g of Na2CO3. What is the value of x?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
2346
views
1
comments
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos