6. Chemical Quantities & Aqueous Reactions
Molarity

Get help from an AI Tutor
Ask a question to get started.
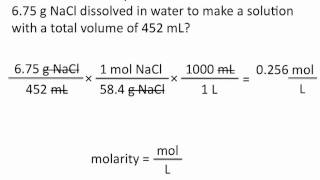
Problem 49b
Textbook Question
Textbook QuestionThe density of acetonitrile 1CH3CN2 is 0.786 g>mL and the density of methanol 1CH3OH2 is 0.791 g>mL. A solution is made by dissolving 22.5 mL of CH3OH in 98.7 mL of CH3CN. (c) Assuming that the volumes are additive, what is the molarity of CH3OH in the solution?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
868
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 14 videos