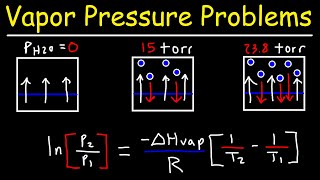
13. Liquids, Solids & Intermolecular Forces
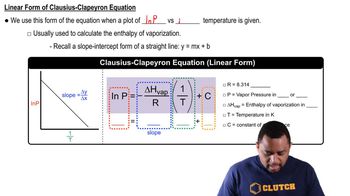
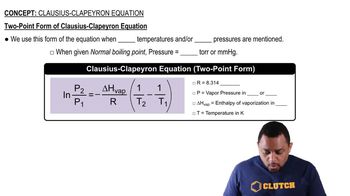
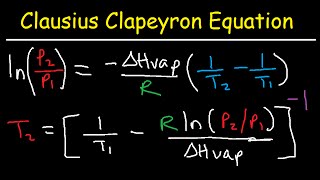
Clausius-Clapeyron Equation

Get help from an AI Tutor
Ask a question to get started.
Problem 93
Textbook Question
Textbook QuestionThe vapor pressure of ethanol (C2H5OH) at 19 °C is 40.0 torr. A 1.00-g sample of ethanol is placed in a 2.00 L con- tainer at 19 °C. If the container is closed and the ethanol is allowed to reach equilibrium with its vapor, how many grams of liquid ethanol remain?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
976
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos