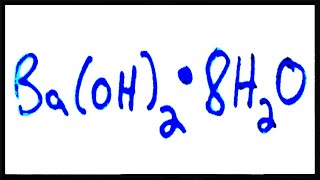3. Chemical Reactions
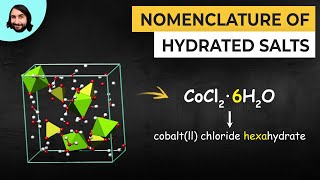
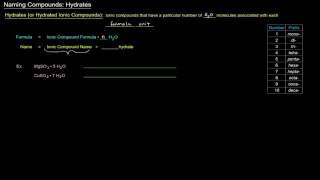
Naming Ionic Hydrates

Get help from an AI Tutor
Ask a question to get started.
Problem 133
Textbook Question
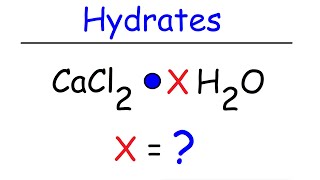
Textbook QuestionEpsom salts is a hydrated ionic compound with the following formula: MgSO4 # x H2O. A 4.93-g sample of Epsom salts is heated to drive off the water of hydration. The mass of the sample after complete dehydration is 2.41 g. Find the number of waters of hydration (x) in Epsom salts.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
4295
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos