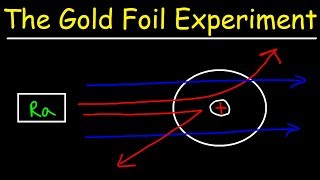
2. Atoms & Elements
Rutherford Gold Foil Experiment

Get help from an AI Tutor
Ask a question to get started.
Problem 40a
Textbook Question
Textbook QuestionMass spectrometry is more often applied to molecules than to atoms. We will see in Chapter 3 that the molecular weight of a molecule is the sum of the atomic weights of the atoms in the molecule. The mass spectrum of H2 is taken under conditions that prevent decomposition into H atoms. The two naturally occurring isotopes of hydrogen are 1H (atomic mass = 1.00783 u; abundance 99.9885%) and 2H 1atomic mass = 2.01410 u; abundance 0.0115%2. (c) Which peak will be the largest, and which the smallest?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
649
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos