11. Bonding & Molecular Structure
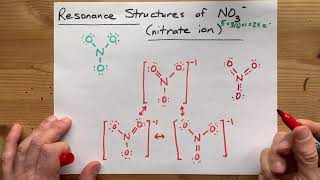
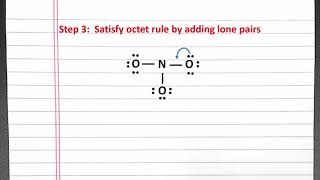
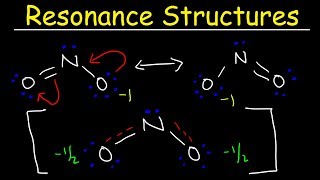
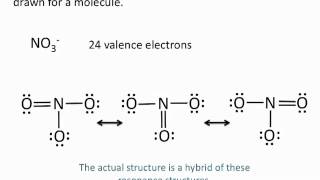
Resonance Structures

Get help from an AI Tutor
Ask a question to get started.
Problem 103
Textbook Question
Textbook QuestionThe structure of borazine, B3N3H6, is a six-membered ring of alternating B and N atoms. There is one H atom bonded to each B and to each N atom. The molecule is planar. (a) Write a Lewis structure for borazine in which the formal charge on every atom is zero.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
1015
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos