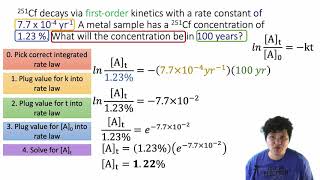
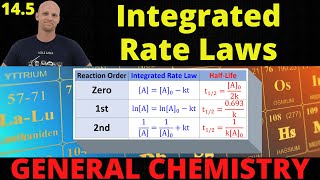
15. Chemical Kinetics
Integrated Rate Law

Get help from an AI Tutor
Ask a question to get started.
Problem 141
Textbook Question
Textbook QuestionFor the thermal decomposition of nitrous oxide, 2 N2O1g2S 2 N21g2 + O21g2, values of the parameters in the Arrhenius equation are A = 4.2 * 109 s-1 and Ea = 222 kJ>mol. If a stream of N2O is passed through a tube 25 mm in diameter and 20 cm long at a flow rate of 0.75 L/min at what temperature should the tube be maintained to have a partial pressure of 1.0 mm of O2 in the exit gas? Assume that the total pressure of the gas in the tube is 1.50 atm.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
12mPlay a video:
601
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos