15. Chemical Kinetics
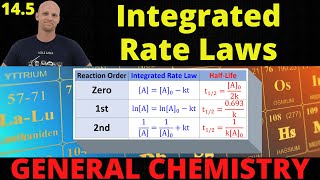
Integrated Rate Law

Get help from an AI Tutor
Ask a question to get started.
Problem 139
Textbook Question
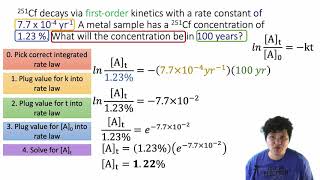
Textbook QuestionThe rate constant for the decomposition of gaseous NO2 to NO and O2 is 4.7>1M ~ s2 at 383 °C. Consider the decomposition of a sample of pure NO2 having an initial pressure of 746 mm Hg in a 5.00 L reaction vessel at 383 °C. (c) What is the mass of O2 in the vessel after a reaction time of 1.00 min?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
10mPlay a video:
934
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos