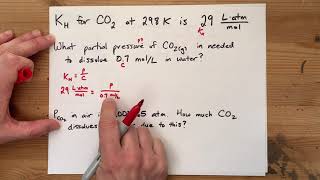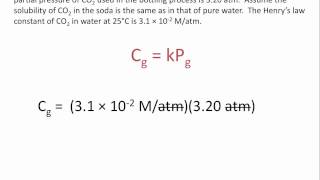14. Solutions
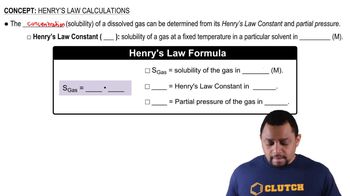
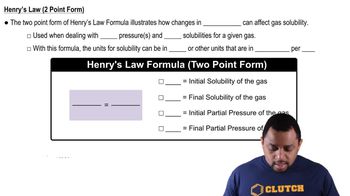
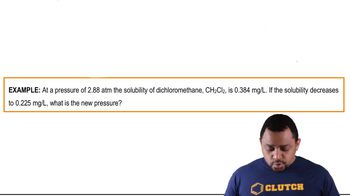
Henry's Law Calculations

Get help from an AI Tutor
Ask a question to get started.
Problem 107
Textbook Question
Textbook QuestionAt ordinary body temperature 137 °C2, the solubility of N2 in water at ordinary atmospheric pressure (1.0 atm) is 0.015 g>L. Air is approximately 78 mol % N2. (b) At a depth of 100 ft in water, the external pressure is 4.0 atm. What is the solubility of N2 from air in blood at this pressure?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
1035
views
1
comments
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos