10. Periodic Properties of the Elements
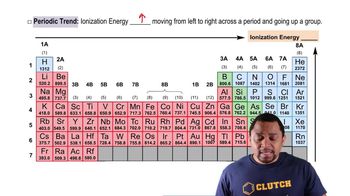
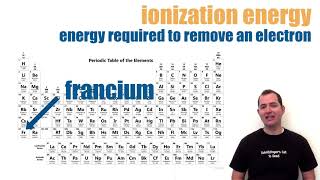
Periodic Trend: Ionization Energy

Get help from an AI Tutor
Ask a question to get started.
Problem 100
Textbook Question
Textbook QuestionThe first ionization energy of the oxygen molecule is the energy required for the following process: O21g2¡O2 +1g2 + e- The energy needed for this process is 1175 kJ>mol, very similar to the first ionization energy of Xe. Would you expect O2 to react with F2? If so, suggest a product or products of this reaction.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
3240
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 14 videos