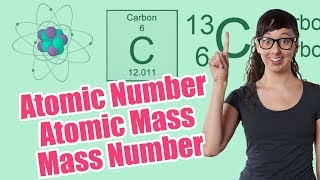2. Atoms & Elements
Atomic Mass

Get help from an AI Tutor
Ask a question to get started.
Problem 120a
Textbook Question
Textbook QuestionMagnesium has three naturally occurring isotopes: 24Mg (23.985) with 78.99% abundance, 25Mg (24.986) with 10.00% abundance, and a third with 11.01% abundance. Look up the atomic weight of magnesium, and then calculate the mass of the third isotope.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
4647
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 16 videos