17. Acid and Base Equilibrium
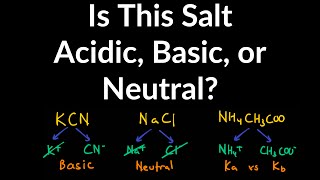
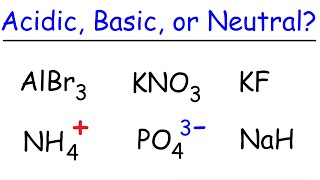
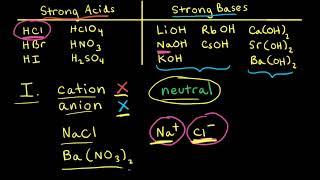
Ionic Salts

Get help from an AI Tutor
Ask a question to get started.
Problem 132
Textbook Question
Textbook QuestionThe hydrated cation M1H2O26 3 + has Ka = 10-4, and the acid HA has Ka = 10-5. Identify the principal reaction in an aqueous solution of each of the following salts, and classify each solution as acidic, basic, or neutral. (a) NaA
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
223
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 14 videos