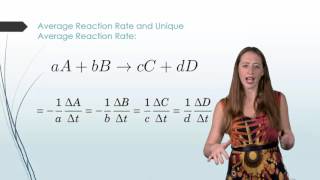15. Chemical Kinetics
Average Rate of Reaction

Get help from an AI Tutor
Ask a question to get started.
Problem 22b
Textbook Question
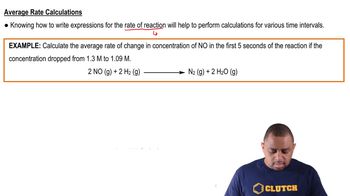
Textbook QuestionThe rate of disappearance of HCl was measured for the following reaction: CH3OH1aq2 + HCl1aq2¡CH3Cl1aq2 + H2O1l2 The following data were collected: Time (min) 3HCl 4 1M2 0.0 1.85 54.0 1.58 107.0 1.36 215.0 1.02 430.0 0.580 (a) Calculate the average rate of reaction, in M>s, for the time interval between each measurement.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
385
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos