14. Solutions
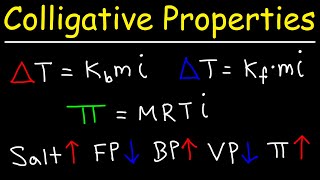
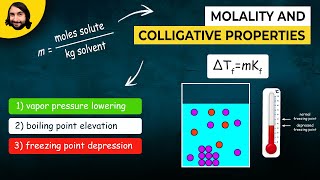
Boiling Point Elevation

Get help from an AI Tutor
Ask a question to get started.
Problem 93
Textbook Question
Textbook QuestionA 1.2 m aqueous solution of an ionic compound with the formula MX2 has a boiling point of 101.4 °C. Calculate the van't Hoff factor (i) for MX2 at this concentration.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
1181
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos