8. Thermochemistry
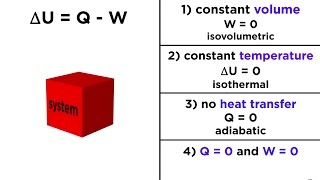
First Law of Thermodynamics -

Get help from an AI Tutor
Ask a question to get started.
Problem 101
Textbook Question
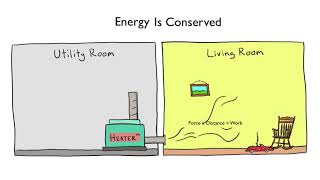
Textbook QuestionConsider a system consisting of the following apparatus, in which gas is confined in one flask and there is a vacuum in the other flask. The flasks are separated by a valve. Assume that the flasks are perfectly insulated and will not allow the flow of heat into or out of the flasks to the surroundings. When the valve is opened, gas flows from the filled flask to the evacuated one. (a) Is work performed during the expansion of the gas? (b) Why or why not?

 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
267
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos









![First Law of Thermodynamics [year-1]](https://img.youtube.com/vi/dHdlH3l8FkM/mqdefault.jpg)