7. Gases
Chemistry Gas Laws: Combined Gas Law

Get help from an AI Tutor
Ask a question to get started.
Problem 48
Textbook Question
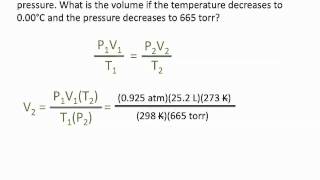
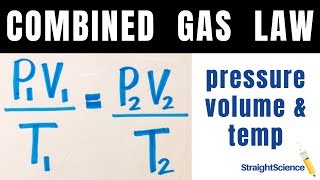
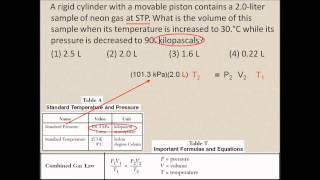
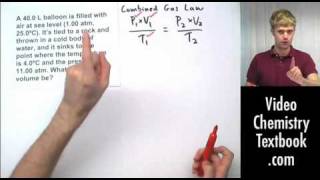
Textbook QuestionAssume that you have a cylinder with a movable piston. What would happen to the gas pressure inside the cylinder if you were to do the following? (d) Halve the Kelvin temperature and triple the volume
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
300
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos