10. Periodic Properties of the Elements
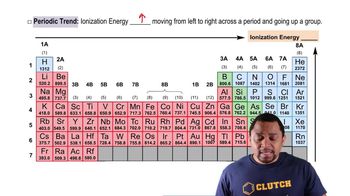
Periodic Trend: Ionization Energy

Get help from an AI Tutor
Ask a question to get started.
Problem 40
Textbook Question
Textbook QuestionIdentify each statement as true or false: (a) Ionization energies are always endothermic. (b) Potassium has a larger first ionization energy than lithium. (c) The second ionization energy of the sodium atom is larger than the second ionization energy of the magnesium atom. (d) The third ionization energy is three times the first ionization energy of an atom.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
57sPlay a video:
492
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 14 videos